
illuminating science
engage
We produce high quality, engaging, and scientifically-accurate materials to instill curiosity and a love of science in people of all ages. From our "science as magic" activity kits to our collections of naturally fluorescent minerals, you'll find something to engage everyone!


educate
Our materials include age-appropriate introductions to the science behind the phenomena. Learn about the science of fluorescent materials from an award-winning scientist and educator!
inspire
Sometimes all it takes to inspire a budding scientist is a quick glimpse of what science can do. Most scientists can tell you what first got them interested in science - from a look through a telescope to a glowing rock. Inspire someone today - with our help!



Adria Updike, founder
Adria has a Ph.D. in physics and worked for NASA before starting her career as a professor in 2011. She is passionate about science and science education, and started this company to share that love of science with as many people as possible.Contact her at [email protected]
Individual, retail, and wholesale options available.
Contact for more information.
Are fluorescent materials safe?
Yes! Fluorescent materials are not dangerous to handle just because they're fluorescent. However, some materials can be both fluorescent and hazardous, so it's a good idea to know a bit about what you're collecting.
Fluorescent Minerals
Many gems and minerals can exhibit fluorescence under the right circumstances. Those circumstances include the inherent chemistry of the mineral itself, small inclusions that may depend on the location in which the mineral was found, the purity and clarity of the mineral, and the wavelength of light shining on the mineral - some fluorescence requires high energy excitation lights than others. In many cases, you can get a number of stones from the same source and some will fluoresce while others don't. That's one of the fun (and often frustrating) aspects of collecting - you never know what you might find!Below, you'll find a collection of photographs I took of minerals in my collection under a range of wavelengths to get an idea for what fluorescence in that mineral might look like and how you might expect to see it.
Hazardous Fluorescent Minerals
Don't eat your rocks! Most common fluorescent minerals require no special instructions for handling and storage, but there are a few you'll want to watch out for. With proper handling and storage, these can be beautiful additions to your collection!In general, it's a good idea not to eat, lick, or inhale particles from your rocks, no matter what they are.Cerussite, a light brown crystal that fluoresces a bright yellow under longwave UV light, contains a high concentration of lead. Lead is a toxin in our bodies, so if you include cerussite in your collection, either wear gloves while handling it or wash your hands afterwards. Don't grind it up or inhale dust from your cerussite.Adamite is a zinc and arsenic-containing mineral that can fluoresce yellow under shortwave and longwave UV. It's fragile and contains toxic levels of arsenic, and dissolves easily in acidic solutions, so if you decide to include it in your collection, store it in a glass box and leave it in there. Don't handle it, break off pieces, attempt to grind or facet it, or breathe in particles that have come off it.Some minerals fluoresce green under longwave or midwave UV light due to a small amount of uranium in the material. Uranium is a naturally-occurring radioactive material found in soil all over the Earth. As it decays, it produces (among other things) radon gas, a carcinogenic gas.Fluorescent minerals containing a tiny amount of uranium include some agates, geodes, chalcedony, adamite, opal, Tiffany Stone, and Hyalite Opal. The amount of uranium in these rocks is tiny and does not present a health hazard; you don't need to take any extra safety precautions when handling these minerals.However, there are some fluorescent minerals with much higher concentrations of uranium that can pose a health hazard. Autunite and Andersonite are examples; Autunite can be half uranium by itself! Uranium emits several types of radiation as it decays, including alpha particles, beta particles, and gamma rays, all of which are dangerous in high enough concentrations. Fortunately, alpha particles can be easily blocked (a sheet of paper will do it - but a glass or acrylic case is even better!) and will block the majority of the beta radiation as well. As for the gamma rays, not much will block those, so your best option is to just not keep it very close to you. Radiation levels drop with the inverse square law, so simply moving away from it can be the best protection. Don't store these materials close to where you eat, sleep, or hang out, and you'll be fine. Keep them in a place with good ventilation (doors and windows that open regularly) and you won't have to worry about the radon gas either.
Are UV lights safe?
That depends on the light!We generally divide UV light sources into three categories - UVA (longwave UV), UVB (midwave UV), and UVC (shortwave UV). Read up on the science of light to learn about how light is classified and measured! UV light is absorbed by glass, so if you want to permanently light up your collection, aim the UV light source at the minerals and put the lights and minerals behind glass doors for safer viewing.
UVA: Longwave UV (395 nm and 365 nm lights)
Longwave UV ranges from the edge of blue light at 400 nm to 315 nm and has the least energy of the three types of UV light, and thus poses the least danger. Longwave UV light includes the common UV flashlights you'll find around 395 nm and 365 nm and the lights you'll find in tanning beds, UV resin curers, and nail salons. Longwave UV flashlights may or may not be sold with a visible-light blocking filter. UVA radiation can cause wrinkles and may be linked to some types of skin cancers, so it's not a good idea to stare directly into the light source or point it at your skin for long periods of time.
UVB: Midwave UV (310 nm lights)
Midwave UV flashlights made with LEDs are a relatively new addition to the market for fluorescent mineral collectors, and can be more affordable and often show many of the same colors you'd see with a shortwave UV light. UVB light extends from 315 nm down to 280 nm, and UVB lights should come with a visible-light blocking filter (otherwise the visible light also emitted by the LED can overwhelm the dimmer fluorescence). UVB light is higher energy than UVA light and longterm exposure can cause skin and eye cancer, so point the flashlight away from eyes and skin while using.
UVC: Shortwave UV (254 nm lights)
Shortwave UV lights include the UVC wavelength range of 280 nm down to 100 nm (at which point the light becomes x-rays). This is the highest energy category of UV light and thus the most dangerous, as it includes ionizing radiation that can cause cancer. Fortunately, UVC radiation doesn't travel very far through air and is quickly absorbed. UVC light sources need to include a visible-light filter. Don't point UVC light sources at eyes or skin while using them, and if you have a permanent UVC light display for your collection, keep it behind glass - glass also absorbs UV light.
Fluorescence Handbook
Here you can find the basic information covered in the Fluorescence Handbook: information about light and energy, ultraviolet light (UV) light specifically, the basics of fluorescence, safety concerns, and where to look for other fluorescent objects.
Light and Energy
The Electromagnetic Spectrum (or EM spectrum) refers to every kind of light, but we can only see a small portion of it with our eyes. Light is both a wave (like a disturbance in water) and a particle (like a ball flying through the air). In physics, this is known as "wave-particle duality." Sometimes light acts like a wave, and sometimes it acts like a particle.Waves have peaks and valleys. The distance between two peaks is known as the wavelength, and is measured in meters (visible light is measured in nanometers, or nm - a nanometer is 0.000000001 meters!). Light moves through space at 670,000,000 miles per hour, the fastest thing in the universe (30 million meters per second).Light carries energy. When light hits something (like a window or your skin) it can either reflect (bounce off the material), refract (pass through the material), or be absorbed by the material. When light is absorbed, the material gains the energy from the light that hit it. The fluorescent minerals covered in this guide absorb the ultraviolet light energy that hits them.
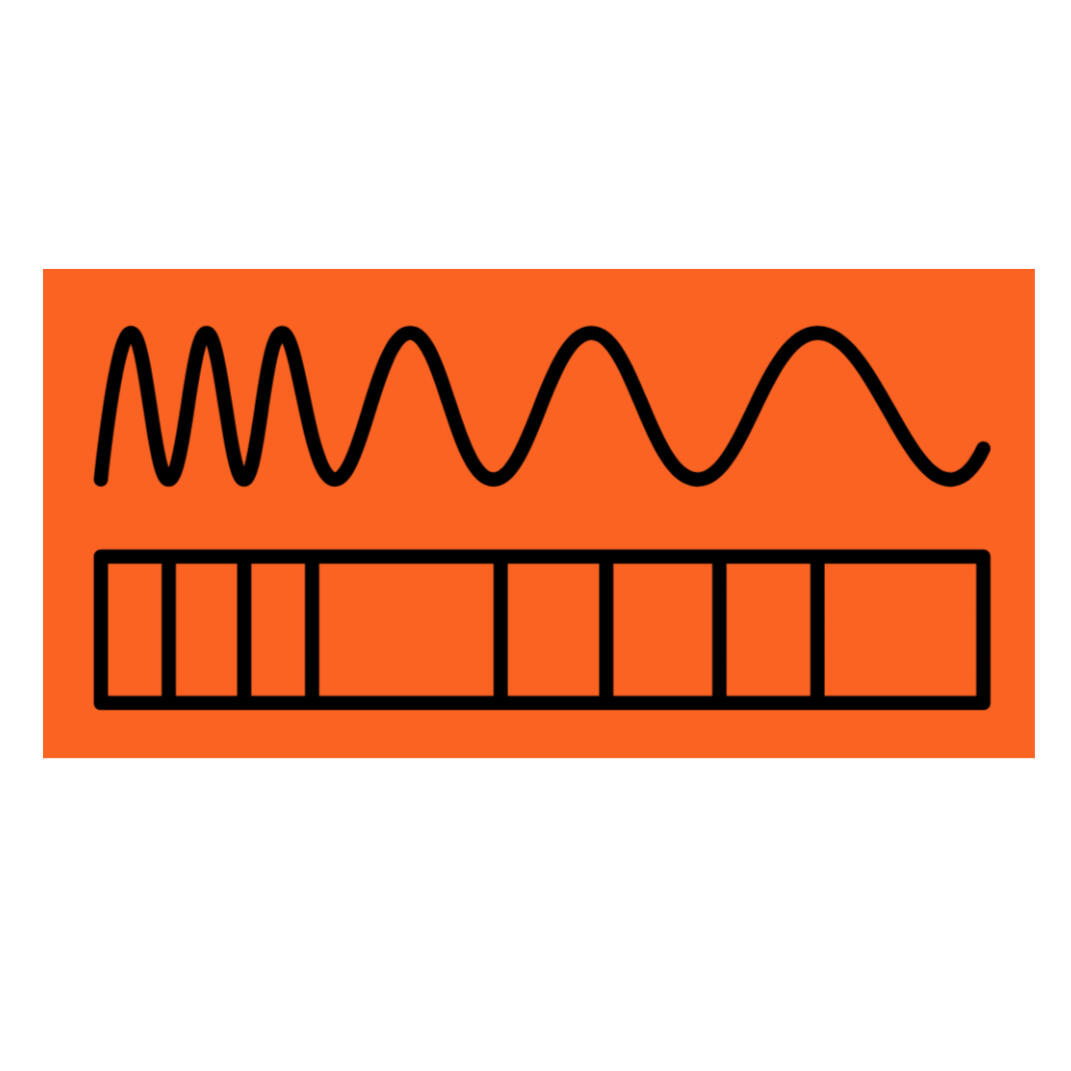
The EM Spectrum
All forms of light are known as "radiation," but not all forms of radiation are harmful - only ionizing radiation is dangerous.The shortest wavelengths of light have the highest energy. The highest energy of light is called gamma rays, followed by x-rays. Both of these are known as "ionizing radiation", which are dangerous to living creatures because they can damage DNA, causing it to make mistakes in replication (mutation).Ultraviolet (or UV) light has less energy than x-rays, but more energy than visible light. UV light sources are used to illuminate fluorescent minerals and glassware, curing resins (like nail polish), and sterilizing materials. Some, but not all UV, is ionizing.All the colors of visible light, from red to violet, fall into a small portion of the EM spectrum between UV and IR light. These are the frequencies human eyes can detect. Visible light and lower energies (below) are non-ionizing.Infrared (or IR) light has less energy and longer wavelengths than visible light. IR light transfers heat - when you stand in sunlight and feel warm, you're absorbing IR light from the Sun.Microwave and radio light have the longest wavelengths and lowest energies. We use them mostly for communications, from your mobile phone to WiFi.
Ultraviolet (UV) Light
UV light is broken up into three categories, all of which can be used as excitation sources to observe fluorescent minerals. Minerals can appear different colors under different excitation wavelengths, and some minerals fluoresce only under specific wavelengths of light.
UV-A Light: 315 - 400 nm (includes 395 nm and 365 nm light sources)
UV-A light (also known as longwave or near-UV) has the lowest energy and is non-ionizing radiation: it won't cause cancer. However, it will cause wrinkles in the long term, so don't point it at your skin for extended periods of time. Most collectors will start out with 395 nm and 365 nm flashlights, as they are the least expensive and illuminate a wide range of materials.
UV-B Light: 280 - 315 nm (includes 305 nm light sources)
UV-B light (also known as mid-UV) is ionizing, so don't look directly into these light sources and don't point them at your skin. Mid-UV light sources are typically the last purchase of a collector, as the advancement in LED technology has only recently made these light sources affordable. Both UV-B and UV-C light sources require filters known as "Woods filters" to block visible light that can overwhelm the mineral fluorescence, which accounts for most of the additional cost.
UV-C Light: 100 - 280 nm (includes 254 nm light sources)
UV-C light (also known as shortwave or far-UV) is the highest energy of UV light. Avoid direct or reflected light from these sources, from fluorescent bulbs or LEDs. Both fluorescent bulbs and LED lights are available for shortwave illumination sources, but they are 5-10 times the price of UV-A lights.
Fluorescence: The Basics
Everything is made up of elements like hydrogen, carbon, and gold. If you keep breaking something in half until you have the smallest possible piece of that material, you’re left with an atom (if it was only made of one element, like a diamond is made of carbon) or a molecule consisting of two or more atoms. Quartz is example of a common mineral compound, a combination of one atom of silicon (Si) and two atoms of oxygen (O), which we label SiO2 (also known as silicon dioxide).Atoms consist of a nucleus (containing protons and neutrons) and electrons orbiting the nucleus. Electrons can orbit at different levels, like floors in a building. Like people standing on floors of a building, electrons orbit at specific levels, not anywhere in between. To move up to a higher level, people need energy to climb the stairs, and the electron needs energy to jump to a higher orbital level. People get their energy by eating food, and the electron gets its energy by absorbing light. The excitation light from your UV flashlight causes electrons to jump up to higher energy levels. But just like people, electrons are lazy, and will eventually drop back down to a lower energy level, emitting light in the process - and that's fluorescence!Fluorescence is typically caused by the addition of an element that doesn’t belong the normal mineral (known as an “impurity” or “activator”), such as a nitrogen atom found in a diamond crystal which normally only contains carbon.
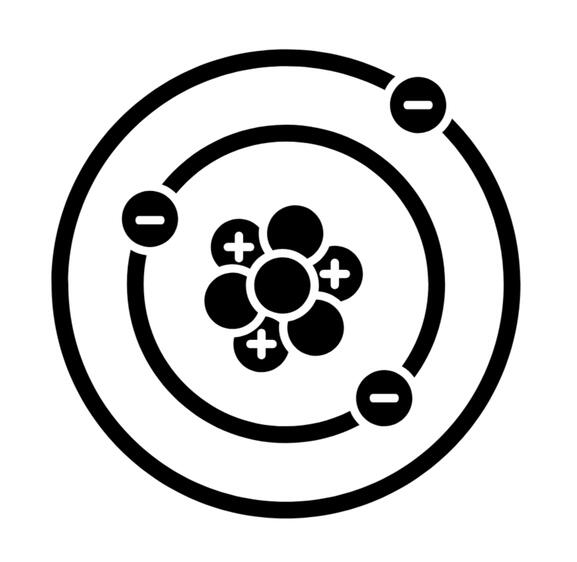
Fluorescence: A Bit More Detail
The energy absorbed in the form of UV light is always more than the energy emitted in the form of visible light through fluorescence. One of the most fundamental principles of physics is that energy is always conserved – the universe doesn't lose or create energy, it just changes from one form to another (ilke the energy in food turning into chemical energy in your body after you eat it). So how can an electron gain energy to jump to higher orbitals, but give up less energy by moving back down?The energy of the light produced in fluorescence is always lower in energy than the light used to excite the electrons (this is known as the “Stokes shift”). When the electron absorbs the UV light and jumps up to a higher level, it will lose a little bit of that energy before jumping back down to the original level. This small amount of energy is transferred to nearby atoms and molecules, causing them to heat up slightly as a result (this is known as vibrational relaxation). Each electron level has a few small sub-levels the electron can be on, each separated by a very small amount of energy.The diagram to the left shows the electron absorbing an ultraviolet photon (the excitation light), jumping up to a higher energy level, relaxing a bit and losing a small amount of the energy it gained, then giving up the rest by jumping back down to the lower level and emitting light (fluorescence) in the process. This is known as a Jablonski Diagram.
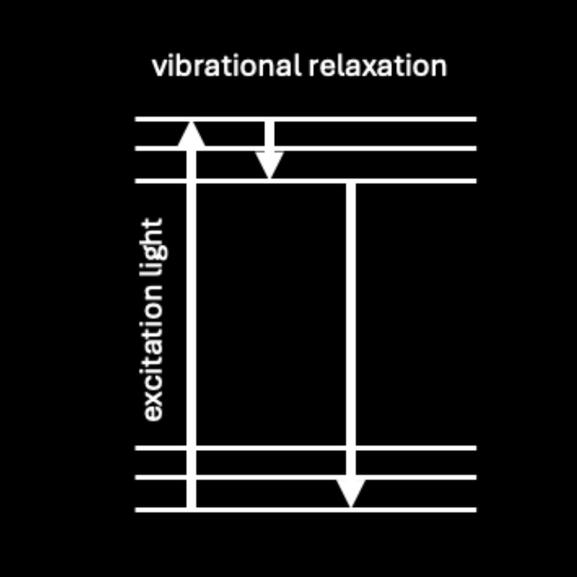
Safety Concerns
Any object that emits particles and/or light is considered "radioactive," but only ionizing radiation is a safety concern. Extended exposure to ionizing radiation can lead to cancer.Not all minerals are perfectly safe to collect (at least in large amounts). Some minerals and glass containing uranium fluoresce a bright green. Uranium also emits ionizing radiation (unrelated to its fluorescence) due to its unstable nature and is found all over the world in relatively small amounts, including the soil in your backyard!The very small amount of uranium found in minerals like agate and opal hyalite makes the radioactivity nearly undetectable by Geiger counters (radiation detectors), but the mineral autunite is nearly 50% uranium by weight and emits much more radiation as a result. If you wish to include autunite in your collection, store it in a well-ventilated area away from areas where people (especially children) spend lots of time.A good rule of thumb - don't eat your mineral collection!
Our Products
Charms Kit
The Charms Kit is an activity kit appropriate for ages 6+. It includes 14 creative, magical pretend play activities using fluorescence as a magical spell.
With the included magic wand, cast the "fluoresce!" spell and watch gemstones, minerals, and even foreign money (yes, they're all real!) light up with hidden colors, patterns, and messages! Write your own secret messages, send them to friends using a papercraft owl, stamp your own images in invisible ink, make up your own stories to go with new constellations, and invent new currency and alphabets among more!